This post is one of a series in the #Innovate4Health policy research initiative.
 By Andrew B. Levey
By Andrew B. Levey
Oxygen therapy, where supplemental oxygen is used as a medical treatment, is vital to children with pneumonia. Rolling blackouts in Uganda and other developing nations, which can last for hours at a time, are stopping oxygen concentrators—machines that concentrate oxygen from the ambient air—from providing this vital therapy. Problems are worse in more remote places that lack an electricity infrastructure in the first place. As a result, children already vulnerable to pneumonia are denied effective treatment. Currently, pneumonia accounts for at least 18% of all deaths of children under 5 years old.
The World Health Organization has stated that methods for providing oxygen are needed in low resource settings to combat poor energy infrastructure and theft of needed supplies. Through their FREO2 Foundation, Professors Roger Rassool and Jim Black of the University of Melbourne are tackling this problem with the FREO2 – Siphon.
FREO2 has improved upon existing oxygen concentrator technology by locally storing oxygen at low pressure, available when needed to supplement a disconnection of energy to the machine. While developing a new approach to the technology, the team managed to keep costs low and produce a product suitable for remote relocations vulnerable to blackouts. Lack of reliable electricity has been addressed in other ways, but those methods are only sufficient for larger, more established medical facilities. More remote and desolate clinics needed a different solution.
Professors Rassool and Black developed a concentrator which utilizes running stream water to create a low-pressure vacuum, which can be used to separate oxygen from the air in the environment. The team determined that the central issue is not specifically energy related, but a matter of creating a difference in air pressure to create air flow. The final product is cost efficient, easy to use, and simple to operate. Taking a step back and re-imagining the basic concepts of an oxygen concentrator allowed the team to arrive at the conclusion that electricity was not needed. A working prototype is already operating in Gippsland, Australia, with planned expansion to other countries. Professor Rassool is conducting operational and field work studies in Uganda to begin implementation of the device where it is needed most.
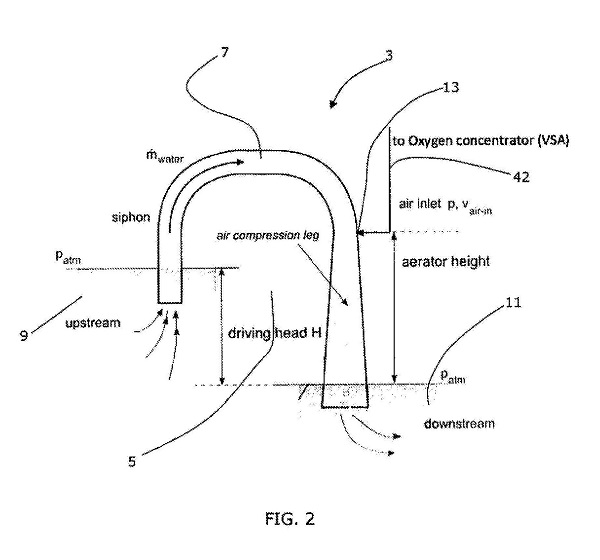
FREO2’s patent application describes the foundational innovation. Concentrating oxygen from the atmosphere involves passing air over a “molecular sieve,” which removes nitrogen and leaves primarily oxygen behind. The energy to move the air across the sieve in older technology was electricity creating high pressure to push air into the sieve. Figure 2 from the patent application shows how a siphon operating between two levels of water can accomplish the same goal by pulling the air instead of pushing. The water flowing through the siphon will draw in air, shown in typical patent fashion with a number (13) and a line. Drawing in air creates a vacuum that can be used to pull air into the sieve and concentrate the oxygen.
The Bill & Melinda Gates Foundation and numerous others have recognized the value of the innovation, awarding the inventors funding to continue research, development, and deployment of their invention. FREO2 is posed to continue its research and manufacturing into the near future. Ultimately, the inventors hope to bring their start up to Australia whereby they would establish a network within the community and potentially form manufacturing capabilities for future deployments. As the inventors note, they have a long road ahead but the rewards to be reaped are great.
The FREO2 device will save lives. As the inventors emphasize, the innovative technology allows remote clinics to keep oxygen concentrators performing by simply utilizing available water sources, which will support their primary mission—saving lives. And the benefits of IP protection will help assure that they fulfill their mission.
#Innovate4Health is a joint research project by the Center for the Protection of Intellectual Property (CPIP) and the Information Technology & Innovation Foundation (ITIF). This project highlights how intellectual property-driven innovation can address global health challenges. If you have questions, comments, or a suggestion for a story we should highlight, we’d love to hear from you. Please contact Devlin Hartline at jhartli2@gmu.edu.
 The problem is even greater in
The problem is even greater in  Thomas J. Shaw, founder of Retractable Technologies, created a technological solution to the problem after seeing a television report of a doctor who contracted HIV from an accidental needlestick. He designed a single use spring-loaded syringe that immediately pulls the needle back inside the just-used syringe. Thus, the needle is automatically covered and incapable of harming anyone.
Thomas J. Shaw, founder of Retractable Technologies, created a technological solution to the problem after seeing a television report of a doctor who contracted HIV from an accidental needlestick. He designed a single use spring-loaded syringe that immediately pulls the needle back inside the just-used syringe. Thus, the needle is automatically covered and incapable of harming anyone. From these initial tests, Shaw founded Retractable Technologies, and the value of his innovation was immediately recognized. He received
From these initial tests, Shaw founded Retractable Technologies, and the value of his innovation was immediately recognized. He received  The value of the retractable syringe design has been important to advancing health care goals worldwide. The World Health Organization has recognized the
The value of the retractable syringe design has been important to advancing health care goals worldwide. The World Health Organization has recognized the  In 2007, Ghanaian tech entrepreneur
In 2007, Ghanaian tech entrepreneur  Through the combination of EarlySensor and Goldkeys, mPedigree’s innovative technology is facilitating the protection of both brand owners and consumers and ensuring that data collection and authentication mechanisms are leading to the safer distribution of medicines, cosmetics, seeds, and other essential products.
Through the combination of EarlySensor and Goldkeys, mPedigree’s innovative technology is facilitating the protection of both brand owners and consumers and ensuring that data collection and authentication mechanisms are leading to the safer distribution of medicines, cosmetics, seeds, and other essential products. By providing a dynamic link between consumers and manufacturers, mPedigree is making communications at the point of purchase routine and creating value for consumers, manufacturers, regulatory agencies, and sellers. A project ten years in the making, mPedigree is built on the recognition that protecting intellectual property—both mPedigree’s and its clients—can save lives.
By providing a dynamic link between consumers and manufacturers, mPedigree is making communications at the point of purchase routine and creating value for consumers, manufacturers, regulatory agencies, and sellers. A project ten years in the making, mPedigree is built on the recognition that protecting intellectual property—both mPedigree’s and its clients—can save lives. One promising example of these new mobile diagnostic applications for eye care is the
One promising example of these new mobile diagnostic applications for eye care is the  How does PEEK work? An app and a clip-on adapter
How does PEEK work? An app and a clip-on adapter