This post is one of a series in the #Innovate4Health policy research initiative.
 By Gabrielle Eriquez
By Gabrielle Eriquez
Because there is currently no preventative vaccine for Zika, a mosquito-borne virus known to cause severe birth defects in pregnant women, the ability to obtain a fast and accurate diagnosis is critical. However, especially in the developing world where Zika’s presence is greatest, there are significant issues with current diagnostic tests: they are in extremely high demand, especially during the summer months, and, accordingly, are very costly.
Enter Nanobiosym’s Gene-RADAR: a tablet-sized device which can detect Zika RNA from human serum. Though it is currently only authorized for Zika testing pursuant to an FDA Emergency Use Authorization, this device has the potential to facilitate the availability of faster, cheaper Zika testing worldwide.
Due to the scale of the disease, getting tested for Zika is not as simple as a quick trip to a local clinic. Testing criteria prioritize pregnant women who have possibly been exposed to the virus. These criteria result in many others who are not pregnant, but still may have been exposed, being turned away from getting tested.
 The problem is even greater in developing countries in Latin America. Poor areas lacking adequate sanitation and air conditioning are favorable breeding grounds for mosquitoes. The only advice that many of these countries give to women to combat Zika is to avoid pregnancy; however, these countries have the world’s highest proportion of unintended pregnancies.
The problem is even greater in developing countries in Latin America. Poor areas lacking adequate sanitation and air conditioning are favorable breeding grounds for mosquitoes. The only advice that many of these countries give to women to combat Zika is to avoid pregnancy; however, these countries have the world’s highest proportion of unintended pregnancies.
The difficulties in preventing and combating Zika that impoverished people in Brazil face have been well documented. For those living in more affluent areas, Zika testing is available even for patients who are not pregnant. But at free public clinics in poorer neighborhoods (where it is easier to contract Zika), lines are out of the door. Symptomatic patients spend hours waiting, only to receive saline for dehydration and to still have to return if their symptoms persist.
The lack of available testing for many patients in developing countries is influenced by cost. Tests are typically hundreds of dollars. For those who can’t even afford window screens or insect repellant, affording a Zika test at this price is next to impossible.
Timing is the other likely factor contributing to this issue. Dr. Anita Goel, CEO and founder of Nanobiosym, noted that even in Florida, testing was back-logged due to medical centers having to ship patients’ samples to outside labs. Results could take up to five weeks to come back.
Outside the U.S., 4 billion people don’t even have access to this basic, albeit inefficient, centralized testing mechanism. “[In developing countries], clinical testing is offered by the occasional network of unregistered laboratories operating without regulatory oversight. Services might be of too poor quality to be of any worth in medical decision-making.” Timing is obviously crucial for pregnant women, but it is also important for any other potentially-infected person, since Zika can be transmitted sexually whether or not symptoms are present.
Gene-RADAR has the potential to remedy these issues by decentralizing and mobilizing testing, thus lowering cost and wait time. Gene-RADAR employs nanobiophysics to diagnose in real time diseases that contain DNA or RNA.
This foundational technology is not limited to Zika. The mobile device was an award nominee for Saving Lives at Birth’s 2015 Grand Challenge for Development for utilizing the platform to detect early HIV in infants in Rwanda. It was also presented in the same year as a diagnostic solution for other global pandemics such as Ebola.
Centralized lab platforms can run from several hundred thousand to one million dollars. Though Gene-RADAR’s cost is still being optimized, the goal is to make it affordable even to the poorest areas of the globe. In terms of wait time, Gene-RADAR should be able to return results in about an hour, eliminating the back-log problem that comes with centralized testing mechanisms.
Gene-RADAR is patented, and does not require running water, constant electricity, or highly-trained personnel to operate. The patented improvements over previous technologies both result in a smaller machine and improvements in the accuracy of testing.
The device’s footprint is much smaller than that of large, centralized testing machines. Gene-RADAR is tablet-sized and only 3.5 pounds, versus 50-plus-pound platforms that are certainly not mobile and likely do rely on constant electricity.
Gene-RADAR diagnosis is also more accurate than that of the other Zika tests currently available. Current testing methods that look for Zika-specific antibodies have a high proportion of false positives. Other tests, like Gene-RADAR, look for DNA or RNA. But these other tests also result in false positives by confusing a sequence with that of another Zika-like virus, such as Dengue. The advances in Gene-RADAR improve accuracy to solve these problems by detecting a virus’s precise RNA sequence.
From a public health perspective, testing as many people as possible in at-risk areas will help contain the virus. If people know quickly whether they’re infected, there is less of a chance of infecting others. Through multiple global initiatives, Nanobiosym’s next step is to increase production and distribution where the need is greatest.
According to Dr. Goel, patent protection via the Nanobiosym incubator has allowed this revolutionary technology to expand beyond the research labs. “Our incubator focuses on bringing together a holistic approach using physics, medicine, nanotechnology, and information technology to create new science or technology, then incubate it all into different products and spin-off companies that can transform how we solve some of the world’s greatest challenges.”
The innovative technology that is Gene-RADAR is a prime example of innovation working to promote groundbreaking solutions to real-world challenges. For Zika (and other diseases with genetic footprints), this means the potential for cheaper, faster, and more readily-available testing that would undoubtedly benefit global health.
#Innovate4Health is a joint research project by the Center for the Protection of Intellectual Property (CPIP) and the Information Technology & Innovation Foundation (ITIF). This project highlights how intellectual property-driven innovation can address global health challenges. If you have questions, comments, or a suggestion for a story we should highlight, we’d love to hear from you. Please contact Devlin Hartline at jhartli2@gmu.edu.
 The Global Innovation Policy Center (GIPC) at the U.S. Chamber of Commerce has just released the sixth edition of its International IP Index. Unfortunately, the report finds that the United States is now tied for 12th place in its patent rankings. This is down from 10th place last year, and it’s down from 1st place just two years ago. The recent downward trend of the U.S. innovation economy is rather alarming, and it is further evidence that the U.S. is quickly abandoning its gold standard patent system.
The Global Innovation Policy Center (GIPC) at the U.S. Chamber of Commerce has just released the sixth edition of its International IP Index. Unfortunately, the report finds that the United States is now tied for 12th place in its patent rankings. This is down from 10th place last year, and it’s down from 1st place just two years ago. The recent downward trend of the U.S. innovation economy is rather alarming, and it is further evidence that the U.S. is quickly abandoning its gold standard patent system.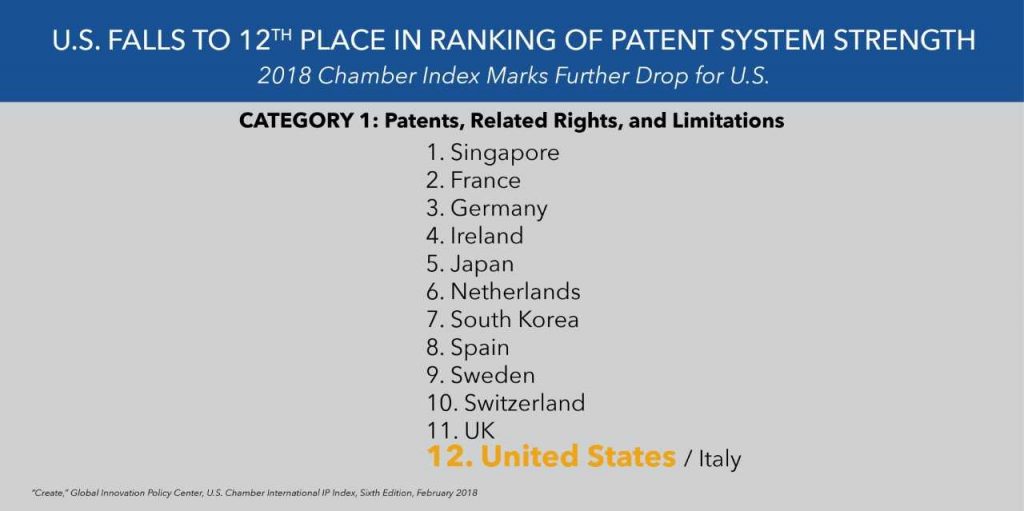
 By Nick Churchill
By Nick Churchill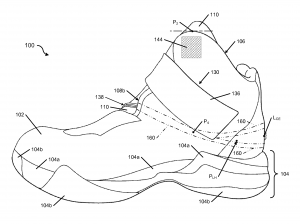 The letter from Walzer explaining the challenges he faced made its way to Tobie Hatfield, the Senior Director of Athlete Innovation at Nike, who
The letter from Walzer explaining the challenges he faced made its way to Tobie Hatfield, the Senior Director of Athlete Innovation at Nike, who 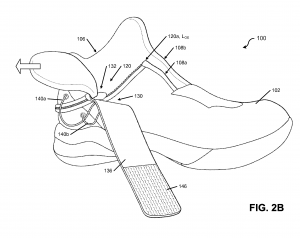 The Nike Ease Challenge
The Nike Ease Challenge  On December 4, 2017, CPIP Founder
On December 4, 2017, CPIP Founder  Miriam achieves its goals through a
Miriam achieves its goals through a 
 On August 14, 2017, the
On August 14, 2017, the  The problem is even greater in
The problem is even greater in  Today, Senators Chris Coons, Tom Cotton, Dick Durbin, and Mazie Hirono
Today, Senators Chris Coons, Tom Cotton, Dick Durbin, and Mazie Hirono