 Over the past ten years, the United States patent system has been transformed by new legislation, regulatory actions, and numerous decisions by the Supreme Court addressing nearly every area of patent doctrine. The many disruptive legal changes have affected infringement remedies, licensing activities, and what types of inventions and discoveries are eligible for patent protection, resulting in a profound sense of uncertainty for most stakeholders. This current state of doubt about the American patent system is pushing investors to look outside of the US for less risky ventures. And because investors are shifting their focus overseas, foreign countries are for the first time poised to bypass the US as the forerunners of innovation.
Over the past ten years, the United States patent system has been transformed by new legislation, regulatory actions, and numerous decisions by the Supreme Court addressing nearly every area of patent doctrine. The many disruptive legal changes have affected infringement remedies, licensing activities, and what types of inventions and discoveries are eligible for patent protection, resulting in a profound sense of uncertainty for most stakeholders. This current state of doubt about the American patent system is pushing investors to look outside of the US for less risky ventures. And because investors are shifting their focus overseas, foreign countries are for the first time poised to bypass the US as the forerunners of innovation.
Last month, the United States Patent & Trademark Office (USPTO), along with the University of Texas Law School and Antonin Scalia Law School, George Mason University, hosted the 12th annual Advanced Patent Law Institute in Alexandria, Virginia. The program featured a distinguished panel of patent experts discussing “current issues around patenting, licensing, enforcing, and monetizing patents in the U.S., and look[ing] at what the UK, EU, and China are experiencing and the impact on U.S. patent practice.” Titled The Current Patent Landscape in the US and Abroad and focusing on the economic factors that spur invention, the consensus was that dramatic changes to the US patent system are driving investment in research and development outside the country and threatening the future of American innovation.
US Patent System No Longer Adequately Incentivizes Investment
Serving as co-moderator with the Hon. Paul R. Michel, Robert Sterne—a leading patent attorney and founding partner of Sterne, Kessler, Goldstein & Fox—kicked off the panel with an overview of a patent system that is falling behind China and the European Union as a driver of innovation. Questioning the Supreme Court’s radical distortion of patent law over the last ten years and the institution of post-grant review, Sterne pointed out that the Patent Trial and Appeals Board (PTAB) has produced over 6,000 proceedings, with patent owner success rates hovering between a meager 30 to 40%. Because of these discouraging numbers, and because injunctive relief has become almost impossible to obtain for patent owners, Sterne warned that critical investment in small and medium-sized companies and universities is rapidly declining.
Judge Michel echoed many of the same sentiments, expressing concern with the “health and vitality and effectiveness of the patent system.” Michel stressed that the principle goal of the patent system is to incentivize investment, but that continued assaults on the system are driving investors to foreign jurisdictions and moving the US in the direction of “off-shore invention.” Citing studies by the Kauffman Foundation and US Census Bureau, Michel explained that most new jobs come from small start-up companies dependent on technology, and that without adequate incentives to invest in these job creators, the patent system and economy are in serious danger.
Expanding on the problem of investment incentivizes, Paul Stone—a partner at venture capital firm 5AM Ventures—discussed his backing over 60 life science startups in the last 15 years, all of which specialized in therapeutics aimed at developing life-saving drugs and drug delivery technologies. Stone offered the following three points to consider regarding the current innovative investment landscape: (1) 60% of the new drugs approved in 2016 came from venture capital-funded small biopharmaceutical companies, not pharma industry giants, (2) of these new approvals, the origin of half the molecules are outside the United States, a much higher percentage than ten years ago, and (3) personalized medicine and the influence of information technology on biotech is leading to smaller market sizes, and a weaker patent system is threatening the ability to realize a return on investments in this area.
Innovation is Moving Overseas
Damon Matteo of Fulcrum Strategy, an IP asset management firm, began his comments with an ominous warning: “Be afraid, be very afraid.” As a practicing IP attorney, Matteo noted that he has seen clients increasingly interested in securing their IP in Europe and China rather than the US, and that China specifically is embracing the software and business method patents that have been abandoned by the US system. Investment has been moving overseas because that’s where patents still have value. Matteo also pointed out that China has been much more favorable to patent owners in IP litigation, as plaintiffs in infringement suits prevail 60% of the time. And injunctive relief—which has become a completely improbable outcome in US litigation—is granted in upwards of 90% of infringement cases in China when there’s been a finding of infringement.
Peter Detkin, founder of the IP development and licensing company Intellectual Ventures, weighed in on some of the “alternative facts” and hysteria that have resulted in the current state of the US patent system. Despite claims over the last 15 years that extortionary demand letters were being sent by the thousands, patent ligation had gotten out of control, and patents were killing investment in R&D and startups, Detkin pointed to multiple analyses by government agencies such as the FTC and the Government Accountability Office that revealed no such exceptional activity. Unfortunately, policymakers took the bait, and entrepreneurs in Silicon Valley have suffered as a result of over-reactive legislative and judicial efforts.
As in-house Chief Intellectual Property Officer of Vivant, a fast-growing home security technology company, Paul Evans provided more insight into how absolutely vital patents are to investments and private equity–backed tech startups, emphasizing how “patents have historically created an important competitive advantage in the marketplace.” Sharing a recent professional anecdote, Evans recounted a conversation with the managing director of a private equity firm with $10 billion in assets in which they discussed the past successful sale of a company based largely on its strong patent portfolio. The two agreed that the transaction would never have happened today due to the immeasurable decline in the value of patents. Evans noted that about 85% of small businesses in the US are now technology based, and that if our patent system can’t protect the inventions they rely on, investments and jobs will be reallocated to jurisdictions that will.
Shifting the discussion to the effect innovation uncertainty is having on universities, patent law and tech transfer expert Chris Gallagher warned that university research funding is at risk, and that the system of grants can no longer be relied upon. Despite a recent case that found the 11th amendment shielded state-chartered schools from IPR exposure, Gallagher encouraged all stakeholders to reach out to Congress to push back on the persistent troll narrative that continues to affect university research.
Efficient Infringement is Devaluing Patents
The panel then moved into a discussion of the increasingly common practice of “efficient infringement,” where companies choose to infringe patents instead of licensing, understanding that the current system has made enforcing patents too expensive and risky. Damon Matteo likened the practice to robbing a bank, getting caught, and as a punishment, only having to return a fraction of the money. Peter Detkin then expanded on the analogy:
It’s a great analogy — the bank robbery — because you not only get to say whether you get caught, but if you get caught, you’ll then be able to argue to the Federal Reserve that the bank really shouldn’t have existed in the first place. Then if that fails, you get to argue to them again that their certificate never should have issued, because it’s a different ground than the first time you argued. Then you could argue that the money was improperly issued to the bank… you have all these administrative ways.
Commenting on efficient infringement, Paul Evans explained that bringing a suit for patent infringement now makes no sense, as the current ecosystem demands high costs to defend patents subject to inter partes review (IPR). According to Evans, the cost of each IPR is between $200,000 and $300,000. IPRs are instituted 70% of the time, and of those cases, 80% of the challenged claims are invalidated. Evans noted that investors are aware of these realities and are hesitant to back certain patent-reliant companies. As a result of the uncertain innovative economy in the US, Peter Detkin noted that patent application filings are down, as well as enforcement actions. Alternatively, countries in Asia and the European Union that have embraced software and biotech patents have seen an increase in filings, enforcement actions, licensing, and investment.
Judge Michel then identified software and health science technology as suffering the most under the current “huge cloud of uncertainty,” and pointed out that China and Europe have broadened patent eligibility in these two tech fields as the US Supreme Court has narrowed it. Michel questioned how anyone could make a eligibility determination given the vague standard set by the Mayo and Alice decisions, and expressed frustration in the Supreme Court’s denial of cert in Sequenom v. Ariosa—a case that would have given the Court an opportunity to correct or at least clarify the Section 101 eligibility analysis. With the Supreme Court unwilling to clean up its mess, Judge Michel expressed support for statutory amendments to 101 recently proposed by the Intellectual Property Owners Association (IPO).
Confidence Must Be Restored in the US Patent System
Wrapping up the panel, Robert Sterne made clear that the patent troll narrative that contributed to so many drastic changes in the US patent system is outdated and no longer relevant. While uncertainty about Section 101 eligibility is ubiquitous, Sterne asserted that “[w]hat is clear is that things are not getting better for innovators in the United States who are relying on the U.S. patent system and who are creating a large bulk of the innovation in our country.” And in addition to losing an edge to foreign jurisdictions in industrial competiveness and job creations, Sterne warned that missing out on innovations in the technology the US employs to protect itself could have dire consequences for national security.
In conclusion, Sterne asked each panelist—as practitioners working in the innovation economy—what they would suggest to bring a sense of confidence back to the bleak patent law landscape. Judge Michel encouraged writing to bring awareness to the situation, including articles, op-eds, and direct letters to members of Congress. Paul Stone urged all stakeholders to focus on quality—specifically on the quality of patents reviewed and the quality of advice given to clients. Damon Matteo suggested adopting a financial mindset that considers the dynamics of returns on investments, which would help stakeholders see patents for the commercial instruments they are and should be. Peter Detkin stressed the importance of relying on hard, verifiable data, not anecdotes and hysteria. Paul Evans discussed the need to create an ecosystem that can be viewed by the investment community with some sense of understanding and confidence. Finally, Chris Gallagher insisted that, no matter the excuses of not having enough time, or not wanting to offend the wrong people, everyone must get involved to insert integrity back into the innovative ecosystem.
The concerns expressed by this panel are being echoed by stakeholders in almost every section of the innovation economy, and without a concerted effort to bring sense and clarity back to the patent system, the US is in danger of losing its competitive and innovative edge.
 CPIP co-founder Adam Mossoff testified on June 13 before the House Judiciary Committee’s subcommittee on the Courts, Intellectual Property and the Internet. He and other witnesses testified about the impact of the Supreme Courts recent decision in TC Heartland LLC v. Kraft Foods Group Brands LLC on innovators and the possibility of future changes to patent law.
CPIP co-founder Adam Mossoff testified on June 13 before the House Judiciary Committee’s subcommittee on the Courts, Intellectual Property and the Internet. He and other witnesses testified about the impact of the Supreme Courts recent decision in TC Heartland LLC v. Kraft Foods Group Brands LLC on innovators and the possibility of future changes to patent law. Unfortunately, much of the discussion centered around a perceived problem with patent “trolls.” This epithet based on myths is often used in the place of reasoned debate for patent policy. As Professor Mossoff explained, as deployed in research and policy debates, this term would make even famous inventors like Thomas Edison a troll. Furthermore, it is both wrong and irresponsible to assume that patent owners who license their inventions are practicing an illegitimate business model. Just as it is perfectly legitimate for a landlord to rent her property instead of selling it, it is likewise perfectly legitimate for a patent owner to license her patent rights instead of manufacturing and selling products to customers. And just as it is legitimate for a landlord to sue a squatter for trespass, it is equally legitimate for a patent owner who licenses her property rights to sue for infringement.
Unfortunately, much of the discussion centered around a perceived problem with patent “trolls.” This epithet based on myths is often used in the place of reasoned debate for patent policy. As Professor Mossoff explained, as deployed in research and policy debates, this term would make even famous inventors like Thomas Edison a troll. Furthermore, it is both wrong and irresponsible to assume that patent owners who license their inventions are practicing an illegitimate business model. Just as it is perfectly legitimate for a landlord to rent her property instead of selling it, it is likewise perfectly legitimate for a patent owner to license her patent rights instead of manufacturing and selling products to customers. And just as it is legitimate for a landlord to sue a squatter for trespass, it is equally legitimate for a patent owner who licenses her property rights to sue for infringement. Over the past ten years, the United States patent system has been transformed by new legislation, regulatory actions, and numerous decisions by the Supreme Court addressing nearly every area of patent doctrine. The many disruptive legal changes have affected infringement remedies, licensing activities, and what types of inventions and discoveries are eligible for patent protection, resulting in a profound sense of uncertainty for most stakeholders. This current state of doubt about the American patent system is pushing investors to look outside of the US for less risky ventures. And because investors are shifting their focus overseas, foreign countries are for the first time poised to bypass the US as the forerunners of innovation.
Over the past ten years, the United States patent system has been transformed by new legislation, regulatory actions, and numerous decisions by the Supreme Court addressing nearly every area of patent doctrine. The many disruptive legal changes have affected infringement remedies, licensing activities, and what types of inventions and discoveries are eligible for patent protection, resulting in a profound sense of uncertainty for most stakeholders. This current state of doubt about the American patent system is pushing investors to look outside of the US for less risky ventures. And because investors are shifting their focus overseas, foreign countries are for the first time poised to bypass the US as the forerunners of innovation. In celebration of World Intellectual Property Day on April 26, 2017, the Center for the Protection of Intellectual Property (CPIP) today joined with the Information Technology and Innovation Foundation (ITIF) to launch “
In celebration of World Intellectual Property Day on April 26, 2017, the Center for the Protection of Intellectual Property (CPIP) today joined with the Information Technology and Innovation Foundation (ITIF) to launch “ The hardest things are often the most important things. That’s one of the implicit justifications for the intellectual property system. If we want people to do the hard and important work of researching, developing, and commercializing game-changing innovations, then we need to secure the fruits of their labor with property rights.
The hardest things are often the most important things. That’s one of the implicit justifications for the intellectual property system. If we want people to do the hard and important work of researching, developing, and commercializing game-changing innovations, then we need to secure the fruits of their labor with property rights.
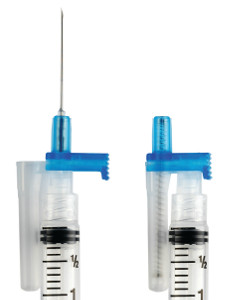 Thomas J. Shaw, founder of Retractable Technologies, created a technological solution to the problem after seeing a television report of a doctor who contracted HIV from an accidental needlestick. He designed a single use spring-loaded syringe that immediately pulls the needle back inside the just-used syringe. Thus, the needle is automatically covered and incapable of harming anyone.
Thomas J. Shaw, founder of Retractable Technologies, created a technological solution to the problem after seeing a television report of a doctor who contracted HIV from an accidental needlestick. He designed a single use spring-loaded syringe that immediately pulls the needle back inside the just-used syringe. Thus, the needle is automatically covered and incapable of harming anyone. From these initial tests, Shaw founded Retractable Technologies, and the value of his innovation was immediately recognized. He received
From these initial tests, Shaw founded Retractable Technologies, and the value of his innovation was immediately recognized. He received 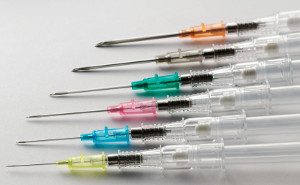 The value of the retractable syringe design has been important to advancing health care goals worldwide. The World Health Organization has recognized the
The value of the retractable syringe design has been important to advancing health care goals worldwide. The World Health Organization has recognized the  On February 16, 2017, CPIP hosted a panel discussion,
On February 16, 2017, CPIP hosted a panel discussion,  On March 8, 2017, CPIP Scholars Adam Mossoff, Devlin Hartline, Chris Holman, Sean O’Connor, Kristen Osenga, & Mark Schultz joined an
On March 8, 2017, CPIP Scholars Adam Mossoff, Devlin Hartline, Chris Holman, Sean O’Connor, Kristen Osenga, & Mark Schultz joined an  The Center for the Protection of Intellectual Property and several of its Senior Scholars are proud to support an
The Center for the Protection of Intellectual Property and several of its Senior Scholars are proud to support an