The following post comes from Colin Kreutzer, a 2E at Scalia Law and a Research Assistant at CPIP.
 By Colin Kreutzer
By Colin Kreutzer
While the vaccines are starting to roll out in the fight against COVID-19, the precise timelines for when they will be widely available continue to be uncertain. But we do have treatments currently available under Emergency Use Authorization authority that have been shown to blunt the impact of the coronavirus and reduce the length of hospital stays. The first one these was Gilead Sciences’ antiviral drug, remdesivir. In July, after an initial period in which Gilead donated its production supply, the company announced a price of $390 per vial, or $2,340 for an estimated 5-day course. While the price is lower than what many analysts were expecting, not everyone was happy about it.
In an August joint letter to HHS Secretary Alex Azar, thirty-four state Attorneys General urged him to do what they contend would resolve a problem of access to the drug: use the “march-in rights” provision of the Bayh-Dole Act to seize Gilead’s patent and license it to generic manufacturers. The response to this proposal from many IP experts can be roughly divided into three main points: (1) it is not legal; (2) it is not effective; and (3) it is dangerously unwise.
What Is Bayh-Dole?
The Bayh-Dole Act of 1980 was a watershed event in the growth of the American pharmaceutical industry. It allowed companies and universities to retain the IP rights to inventions that were developed using government-funded research. The goal was to improve the efficiency with which innovations were brought to market and to encourage investment and collaboration between government, university, and private researchers. Previously, many research developments never saw the light of day due to lack of commercialization, and likely many other inventions were never born in the first place. Bayh-Dole is widely regarded as a success story on both sides of the aisle.
What Are March-In Rights?
Since the aim of the law is to spur innovation and development, the march-in rights provision was included to counteract patent owners who “hold out” or fail to commercialize their inventions. Under very limited circumstances, it allows the government to “march in” and force the owners to license their patent on reasonable terms to a third party. Just how limited are those circumstances? So far, the 40-year-old provision has been used exactly zero times. It stands to reason that valuable products don’t need to be forced into the market, and many modern treatments–for cancer, diabetes and hepatitis, among others–have been invented and commercialized under Bayh-Dole collaborations without any intervention.
It Is Not Legal
Notably absent from the list of Bayh-Dole creations is remdesivir. The law only applies to inventions that are “conceived or actually reduced to practice in the performance of work under a funding agreement,” i.e., things that were invented with government help. It does not apply to every case in which a drug maker has worked alongside a government agency at one stage or another. The AG letter cites $30 million in NIH-funded work on remdesivir. It claims that this funding exposes the drug to the march-in provision. The letter also makes a general appeal to our sense of fairness—the public paid for this, and so it rightly belongs to all of us.
As noted by Stephen Ezell of the Information Technology and Innovation Foundation (ITIF), the total government expenditure is actually closer to $70 million. That number includes additional work performed with USAMRIID, the U.S. Army Medical Research Institute of Infectious Diseases. Both projects took place in 2014. The Army study was investigating Gilead’s library of antiviral compounds for effective Ebola treatments. Remdesivir’s compound gave positive results, but other treatments proved better. The NIH project was a clinical trial to explore whether remdesivir could be used against coronaviruses as a general class. Again, it showed promise. But the relevant coronaviruses at the time (SARS and MERS) did not spread widely enough to make larger studies feasible. The NIH study might have enabled Gilead to home in so quickly on remdesivir as a COVID-19 treatment, and in that sense, it would have played a crucial role. But that is not the same thing as having a hand in the actual invention of the drug.
Critically, under both studies, the drug had already been invented by Gilead. Regarding the NIH work, a HHS spokeswoman told STAT that the department does not consider the march-in rights to apply. And as pointed out by Scalia Law Professor Adam Mossoff, Army lawyers have stated that their contributions did “not qualify USAMRIID as a joint inventor of the compound.” Even if they were joint inventors, the NIH has stated that “the extraordinary remedy of march-in is not an appropriate means of controlling prices.”
As CPIP Executive Director Sean O’Connor explains at The Hill, even if inventorship could be established, march-in rights would still not be legal in this case: “[m]arch-in rights under Bayh-Dole’s Section 203 only authorize the government to grant new licenses if the original funding recipient fails to take steps to bring the invention to the market (achieve ‘practical application’) or reasonably satisfy health or safety needs.”
And yet, while it pales in comparison to the over $1 billion that Gilead expects to spend this year on remdesivir, $70 million sounds like a lot of tax dollars. CPIP Senior Scholar Kristen Osenga argues that proponents of marching in “mislead the public, specifically regarding remdesivir and, more generally and dangerously, regarding government support of scientific research.” She urges people to understand that government collaborations are a great deal for the public, and among the most efficient ways that the government spends our money: “The Milken Institute estimates that the long-term boost to total economic output could be as high as $3.20 for every dollar the NIH invests in biopharmaceutical research. Even conservative estimates peg the value of the NIH at $1.70 of economic activity per dollar spent. If only all government spending were so productive.”
It Is Not Effective
The preceding section alone answers the question of whether Bayh-Dole is a legal means of seizing Gilead’s property rights. Clearly, though, it does not quiet the sentiment felt by many that something else needs to be done. In addition to the price tag, the AG letter speaks of a “dangerously low supply” of the drug. But the letter supports that claim with a dubious comparison of Gilead’s expected production output to every single confirmed case of COVID-19 in the country. It should be clear on its face that this is not a valid manner of determining how many patients would actually benefit from remdesivir. It would have been more appropriate to say that future demand is uncertain. Because of course, supply is only one half of the equation. Demand can vary greatly depending on whether we cooperate as a society to contain this virus.
Gilead has, in fact, licensed the drug to third parties in order to increase supply. Currently, its own production will remain domestic. But Joseph Allen at IPWatchdog notes that in addition to ramping up its own capacity, Gilead has deals with drug makers in Egypt, India, and Pakistan to provide supplies internationally. Mr. Allen is also is a former congressional staffer who worked on the Act with its namesake, Senator Birch Bayh (D-Ind.). He adds that, because march-in seizure is a hostile measure, it would involve a drawn-out legal battle. This would render the process far too slow to be effective in a pandemic.
It Is Dangerously Unwise
Far from being proof in support of the AGs’ position, Gilead’s work with NIH is a clear example of how damaging it would be to abuse the march-in rights provision. We desperately want these types of collaborations to continue. And if companies believe that doing so would expose them to the seizure of their IP, they will act accordingly.
Our intellectual property system provides the necessary incentives for companies to invest massive amounts of money and bring new lifesaving drugs to the world. We even allow patents for new uses of existing drugs. As CPIP Senior Fellow for Life Sciences Chris Holman points out, the next great cure might be hiding in your medicine cabinet. But without incentivizing the R&D expenditures that bring us these wonderful inventions, we may never realize it.
It is hard to worry about the future when the present appears so bleak, but it is critically important to understand why it is dangerous to weaken the incentives that have given us so many lifesaving developments. Even if exercising march-in rights were legal, and even if it could somehow increase production, it is necessary to consider the long-term implications. Taking away a company’s rights and forcing it to sell at close to the cost of production may help with the current situation, but it will likely decimate future research. Who would want to spend billions of dollars on R&D without the knowledge that they can obtain IP rights that will have a predictable value? We should ensure that companies remain strongly incentivized to research new treatments that benefit us all.
 CPIP has published a new policy brief by Joanna M. Shepherd, Vice Dean and Thomas Simmons Professor of Law at Emory University School of Law. The brief, entitled
CPIP has published a new policy brief by Joanna M. Shepherd, Vice Dean and Thomas Simmons Professor of Law at Emory University School of Law. The brief, entitled  By Colin Kreutzer
By Colin Kreutzer The global COVID-19 pandemic has challenged multiple aspects of modern society in a short time. Health and public safety, education, commerce, research, arts, and even basic government functions have had to change dramatically in the space of a couple months. Some good news in all this is the response of many companies in the intellectual property (IP) industries: they are stepping up to make sure crucial information and materials are available to speed research and development (R&D) towards vaccines, therapeutics, and medical devices. This blog post gives a sampling of the current initiatives facilitating the best innovative work the world has to offer.
The global COVID-19 pandemic has challenged multiple aspects of modern society in a short time. Health and public safety, education, commerce, research, arts, and even basic government functions have had to change dramatically in the space of a couple months. Some good news in all this is the response of many companies in the intellectual property (IP) industries: they are stepping up to make sure crucial information and materials are available to speed research and development (R&D) towards vaccines, therapeutics, and medical devices. This blog post gives a sampling of the current initiatives facilitating the best innovative work the world has to offer. In a new CPIP policy brief entitled
In a new CPIP policy brief entitled  By
By 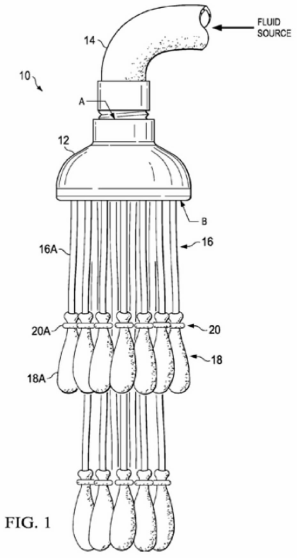
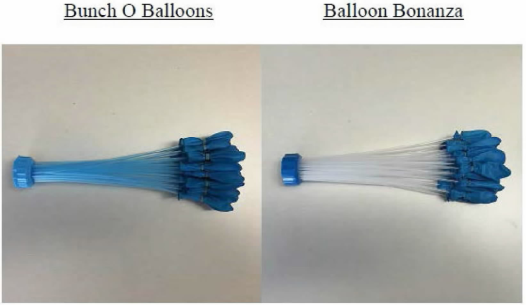
 Last week, a group of CPIP scholars—Chris Holman, David Lund, Adam Mossoff, and Kristen Osenga—filed an
Last week, a group of CPIP scholars—Chris Holman, David Lund, Adam Mossoff, and Kristen Osenga—filed an