By Jack Ring
 In their forthcoming paper, Solutions Still Searching for a Problem: A Call for Relevant Data to Support “Evergreening” Allegations,[1] C-IP2 Senior Scholars Erika Lietzan of Mizzou Law and Kristina Acri of Colorado College call for relevant data to support evergreening allegations and accompanying policy proposals. “Evergreening” is often described as brand drug companies securing additional patents and FDA exclusivities, which grant greater market exclusivity than the initial exclusivities.[2] Evergreening has long been the subject of criticism and policy reform.
In their forthcoming paper, Solutions Still Searching for a Problem: A Call for Relevant Data to Support “Evergreening” Allegations,[1] C-IP2 Senior Scholars Erika Lietzan of Mizzou Law and Kristina Acri of Colorado College call for relevant data to support evergreening allegations and accompanying policy proposals. “Evergreening” is often described as brand drug companies securing additional patents and FDA exclusivities, which grant greater market exclusivity than the initial exclusivities.[2] Evergreening has long been the subject of criticism and policy reform.
The article evaluates empirical data commonly offered to substantiate evergreening and explains that the data, while largely accurate, does not support proposed policy changes. The authors argue that the most relevant data points for policymakers are (1) when brands face competition and (2) what drives the timing of that competition. The authors indicate that no empirical studies answer these questions, so this article concludes by proposing a study designed to properly consider these factors.
I. Background
Evergreening allegations stem from protections on brand drugs that advocates view as too many patents or FDA exclusivities, which, they claim, improperly extend the drug’s exclusivity.[3] FDA exclusivities include exclusive periods of approval or markets as well as processes for bringing generic drugs to market. Under the Federal Food, Drug, and Cosmetic Act (FDCA), the FDA approves all new drugs before they are sold.[4] However, the FDCA does not define “drug” or “new drug,” which may refer to an active ingredient, a finished product, or both.[5] While the FDCA does not specify, the FDA in practice approves products (finished medicines as they are sold in the market), not active ingredients (active molecules and components of finished products).[6]
The FDCA controls the processes of bringing a generic drug to market.[7] As critics point out, some statutory processes bar generic drugs from entering the market until the patents expire. However, this is not always the true.[8] Moreover, the FDCA provides different forms and lengths of exclusive approval as a reward for drug makers performing the preclinical and clinical research needed to bring a drug to market. These range from six months for performing pediatric studies[9] to seven years for “orphan” drugs intended to treat a rare disease or condition.[10]
Much of the evergreening allegations and outcry focus on exclusivities stemming from continuing innovation. Continuing innovation is common because developing new molecular entities is time- and cash-consuming. Therefore, brand companies benefit from identifying new uses for new molecular entities. Moreover, those new medical uses (indications) may be eligible for new patents and statutory exclusivities. Protections for continuing innovation, however, are narrow and only prevent the approval of generic drugs for that new, specific use.[11]
II. The Hastings Project and Current Data for Policymakers
The University of California Hastings College of Law hosts a database that (1) identifies the earliest and latest expiring patent or exclusivity for new drugs and (2) calculates the number of months between those dates.[12] The authors undertook a large audit of the Hastings Database. Like the Hastings Database, major empirical studies offered to support the allegation of “evergreening” focused on counting patents and exclusivities.[13] The Hastings Database utilizes three counting metrics: earliest protection end date, latest protection end date, and delta between the two called “months added.” The authors’ audit raised questions regarding the inferences drawn about competition from patent and exclusivity counts generally.
The authors argue that the Hastings Database is insufficient to inform policy debate because it does not provide the most relevant piece of information for policymakers: when new drugs face competition and why. The Hastings Database estimates new drug entry and competition based on the latest protection date for a drug’s applicable exclusivities. However, the exclusivities used to calculate that date do not prohibit all new drug entry. Therefore, because new drugs could enter the market before the latest protection date, that data point does not serve as a relevant data point for policymakers seeking to drive timely generic competition. In the authors’ own data review, every new chemical examined had a generic drug available before the latest expiry date listed in the Hastings Database. The authors’ audit confirmed their skepticism of the “latest protection end date” as a proxy for the likely generic entry date. Actual generic competition date will likely launch at least five years earlier, with nearly 18% launching more than ten years sooner.[14]
III. Takeaways and the Call for Relevant Data
While the authors audited the Hastings Database and analyzed their own dataset, they recognized their research still did not provide the answers to the most important questions: (1) when do generic drugs reach the market and (2) what drives that timing? A study designed to consider the market entry date of the first generic drug based on any brand product containing a particular new active ingredient would determine the factors driving that market entry date.
The publication closes by describing this better study and calling for this data. At a high level, the study would focus on each new molecular entity approved since 1983 with the relevant dates being the “Initial Protection End Date” and the “NCE Competition Date.” Initial Protection End Date would start with the first approved brand product containing the NCE. NCE Competition Date would be the commercial launch date for the first product, approved on the basis of an abbreviated application (relying on the brand company’s research), to contain that same NCE for the same indication(s). They recommend a database covering all new molecular entities since 1984 to allow policymakers to study these trends. The database would allow policymakers to see exactly how long brand companies with new chemical entities enjoy a market without competition from another company marketing the same chemical entity for the same use on the basis of the brand company’s own research. Where the Generic Competition Date (actual commercial launch date) is later than the Initial Protection End Date, one would need to investigate the reason for its timing. Perhaps the generic company had difficulty making a bioequivalent, the market is too small, or the generic company faced manufacturing issues.
IV. Policy Implications
As the authors make clear, policymaking based on latest expiration date (the Hastings Database approach) before consideration of actual market entry (the authors’ proposed study) would be premature. The number of patents and exclusivities, and the difference between the earliest and latest expiration date of patents and exclusivities, do not illustrate evergreening. Yet, current policy proposals rely on this counting method used by the Hastings Database to support reforms. This is reliance on data to with no correlation to the purported issue. This article, rather, provides a sketch of how a proper database could be built and a study could be conducted to measure evergreening. Evergreening claims can only be substantiated with proper empirical data. Unless empirical data shows that evergreening is a problem, policy solutions are unnecessary.
[1] Erika Lietzan and Kristina Acri née Lybecker, Solutions Still Searching for a Problem: a Call for Relevant Data to Support “Evergreening” Allegations, 33 Fordham Intell. Prop., Medifa & Ent. L.J. (forthcoming 2023), https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4230310#.
[2] For an overview of arguments that drug companies obtain too many patents and too much exclusivity, which raises prices, see Erika Lietzan, The “Evergreening” Metaphor in Intellectual Property Scholarship, 53 Akron L. Rev. 805, 848-851 (2020); see also Erika Lietzan, The Evergreening Myth, Regulation 24, 25 (Fall 2020).
[3] E.g., Robin Feldman & Evan Frondorf, Drug Wars: A New Generation of Generic Pharmaceutical Delay, 53 Harv. J. on Legis. 499, 510 (2016); Michael A. Carrier, A Real-World Analysis of Pharmaceutical Settlements: The Missing Dimension of Product Hopping, 62 Fla. L. Rev. 1009, 1016 (2010).
[4] 21 U.S.C. § 355(a).
[5] The term “drug” is ambiguous at FDA. The FDA approves brand products, not active ingredients, and those products are copied by generic companies. As a result, a brand’s active ingredient may be spread over multiple products. 21 U.S.C. § 321(g).
[6] FDA defines “active ingredient” as “any component that is intended to furnish pharmacological activity or other direct effect in the diagnosis, cure, mitigation, treatment, or prevention of disease, or to affect the structure or any function of the body of man or other animals.” 21 C.F.R. § 314.3(b). The active ingredient includes the ester, salt, or other noncovalent derivative of the molecule responsible for the physiological or pharmacological action of the drug substance. 21 C.F.R. § 314.3(b). That molecule, in turn, is the “active moiety.”
[7] See 21 U.S.C. §§ 355(j)(2)(A)(vii)–(viii), 355(j)(2)(B)(i).
[8] These circumstances include when (1) the patent claims a method of use for which the generic company does not seek approval, or (2) the brand company does not sue for patent infringement after a paragraph IV certification. 21 U.S.C. §§ 355(j)(2)(A)(vii)(IV); id. § 355(j)(2)(B)(i).
[9] 21 U.S.C. § 355a. Pediatric exclusivity is awarded after the research is complete, when the brand company submits a report to the agency that “fairly” responds to the written request. Id. § 355a(d)(4).
[10] Id. § 360bb(a)(2).
[11] Moreover, generic companies seeking to enter the market can choose not to seek approval for the new indication. 21 C.F.R. § 314.127(a)(7). For example, if a brand drug treats conditions A, B, and C and condition C is still subject to a patent or statutory exclusivity, a generic drug company could still receive approval to sell their drug to treat condition A and B.
[12] See Evergreen Drug Patent Search, https://sites.uchastings.edu/evergreensearch.
[13] This includes pieces by Robin Feldman, a Hastings professor. Robin Feldman, May Your Drug Price be Evergreen, 5 J.L. & Biosci. 590, 590 (2018); Amy Kapczynski et al., Polymorphs and Prodrugs and Salts (Oh My!): An Empirical Analysis of “Secondary” Pharmaceutical Patents, 7 PLOS Online 12 (2012).
[14] Lietzan & Acri, supra note 1, at 44–46.
 I. INTRODUCTION
I. INTRODUCTION By Tabrez Ebrahim
By Tabrez Ebrahim By Wade Cribbs
By Wade Cribbs
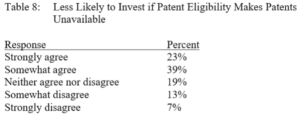

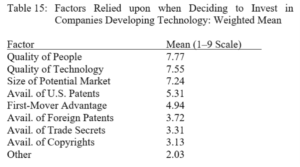
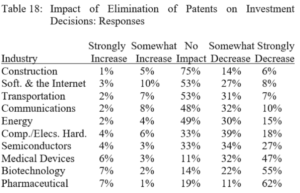
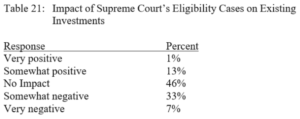

 By Austin Shaffer
By Austin Shaffer By Yumi Oda
By Yumi Oda By Colin Kreutzer
By Colin Kreutzer