Key Research Focus:
- Enzyme production for hydrolysis applications
- Application driven enzyme mixtures
- Process design improvement and reduced costs
Enzymes offer unique “green” options for many common industrial processes. These inherently gentle bio-derived chemicals can replace or enhance many of the more classic synthetic processes. Our group has worked on direct application and production of enzymes to accomplish industrial tasks. Much of our study has focused on producing hydrolytic cellulases, xylanases, pectinases, arabinases and lactases. These enzymes (or enzyme groups) can be applied to a wide array of applications including paper pulp wastewater treatment, cellulosic ethanol feedstock preparation, or soy protein isolation.
Understanding the cellular response to different stimuli is paramount for successful enzyme production. By carefully understanding the enzyme mixtures required for successful substrate hydrolysis, the fungal growth can be tailored to ensure a correct product composition. Our group has recently applied this forward thinking approach to both guayule and soy carbohydrate hydrolysis.
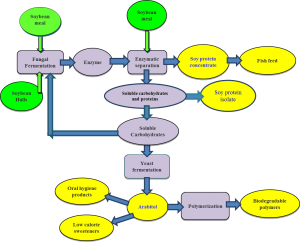
Enzymes for Soy Protein Isolation
Soy protein is one of the major components of the diet of food producing animals and is increasingly important in the human diet as well. However, soy protein cannot be used as an ideal protein supplement in foods, because of the presence of high amount of indigestible carbohydrates in the soybean meal. To enhance the nutritional value of soybean meal in human food and animal feed, it is necessary to improve the protein content and remove the indigestible carbohydrates from the soybean meal during the processing of soy protein diets. This project aims to develop economically feasible technologies and processes for separating, enriching and upgrading soy proteins and carbohydrates from soybean meal. The objective is to separate proteins from carbohydrates (and other minor components) in soybean meal, facilitated by enzymatic hydrolysis of poly- and oligo-meric carbohydrates and other non-protein materials. The enriched proteins obtained are valuable for high-quality feed, food and industrial uses. The hydrolyzed carbohydrates could also be converted via fermentation into bio-fuel and other value added products.
By using a complex mixture of enzymes, our group has designed a process (Patent App #WO2013170017) to produce soy protein concentrates and isolates without costly ethanol or complex pH schemes. This research has worked to understand how different enzymes are created by different microorganisms and their overall activity for successful protein isolation. By carefully optimizing both fermentation and hydrolysis conditions, an economically guided study has established a reliable method to use natural enzymes to produce enhanced soy products.
Cellulase Production for Biofuels
Cellulase is a group of enzymes that hydrolyzes cellulose to glucose. This enzyme complex is most commonly produced by fermentation of the fungi Trichoderma reesei. However, enzyme production can be subject to complex metabolic regulation by both substrate induction and glucose repression. While the intermediates of cellulose hydrolysis provide effective induction, the accumulating glucose product represses the cellulase synthesis.
We have proposed a coupled fermentation and foam recovery process (Patent App #WO2006119048 A2) that can maintain optimal cellulase concentrations in the fermentation broth by continuously removing the protein product produced. At optimal cellulase concentrations, enough glucose is generated for cell maintenance and active cellulase synthesis and yet, avoids product concentrations that could cause the fungus to reduce production.
Foam Fractionation Technology
This project is aimed at developing an innovative cellulase production process by coupling fermentation with in-situ foam fractionation. Foam fractionation, merely bubbling air through the production broth, is simple, energy-efficient, and environment-friendly when compared with conventional methods (salt/solvent precipitation, adsorption/chromatography). This innovative process is advantageous to avoid cellulase deactivation caused by shear and proteases present in the fermentation process. The continuous removal of cellulase by foam fractionation minimizes its exposure to these negative effects. Furthermore, higher specific productivity can be achieved by maintaining optimal cellulase concentrations for balanced glucose generation (by cellulose hydrolysis) and consumption, while retaining the cells and solid substrate in the fermentor.
T. reesei Pellet Technology
Many different approaches have been suggested to ensure that fungal cells remain in the fermentor during enzyme production. Most research as focused on substrate immobilization to anchor the mycelial fungal structure to a more process compatible surface. However, our approach has been to influence the fungus to produce compact pellets. This process effectively eliminates the need for immobilizing substrates. Whether coupled with cellulase foam collection or fed batch systems, the enzyme producing cells can be retained to reduce the need to re-grow the population after each harvest (Patent App# 20110027858)
 |
 |
| T. reesei Rut C-30 naturally growing as filaments in an agitated fermentor. | T. reesei Rut C-30 made to grow as pellets in an agitated fermentor. |
Publications:
Effect of Natural and Pretreated Soybean Hulls on Enzyme Production by Trichoderma reesei. (A. M. Coffman, Q. Li, L.-K. Ju) Journal of the American Oil Chemists’ Society 91 (8), 1331-1338 (2014). View Article
Promoting Pellet Growth of Trichoderma reesei Rut C30 by Surfactants for Easy Separation and Enhanced Cellulase Production. (N.V. Callow and L.-K. Ju) Enzyme and Microbial Technology 50(6-7), 311-317 (2012). View Article
Cellulase production by continuous culture of Trichoderma reesei Rut C-30 using acid hydrolysate prepared to retain more oligosaccharides for induction. (C.-M. Lo, Q. Zhang, N. V. Callow and L.-K. Ju) Bioresource Technology. 101(2), 717-23 (2010). View Article
Cell immobilization with polyurethane foam for retaining Trichoderma reesei cells during foam fractionation for cellulase collection. (Q. Zhang, C.-M. Lo, and L.-K. Ju) Applied Biochemistry and Biotechnology 156, 12-23 (2009). View Article
Cellulase production by cocultures of Hypocrea jecorina Rut C30 and Candida bombicola. (C.-M. Lo and L.-K. Ju) Enzyme and Microbial Technology 44(2), 107-111 (2009). View Article
Factors affecting foaming behavior in cellulase fermentation by Trichoderma reesei Rut C-30. (Q. Zhang, C.-M. Lo, and L.-K. Ju) Bioresource Technology 98(4), 753-760 (2007). View Article
Affinity foam fractionation of Trichoderma cellulase. (Q. Zhang, C.-M. Lo and L.-K. Ju) Applied Biochemistry and Biotechnology 129-132, 1051-1065 (2006). View Article
Cellulase production by Trichoderma reesei using sawdust hydrolysate. (C.-M. Lo, Q. Zhang, P. Lee, and L.-K. Ju) Applied Biochemistry and Biotechnology 121-124: 561-573 (2005). View Article
Wastepaper hydrolysate as soluble inducing substrate for cellulase production in continuous culture of Trichoderma reesei. (L.-K. Ju and O. A. Afolabi) Biotechnology Progress 15, 91-97 (1999). View Article
Enhancing enzymatic saccharification of waste newsprint by surfactant addition. (J. Wu and L.-K. Ju) Biotechnology Progress 14, 649-652 (1998). View Article