Biofouling and biofilm formation are ubiquitous in nature and have significant environmental and industrial impacts. For maritime industries, biofouling increases fuel consumption and maintenance costs of marine vessels. Approximately 80% of the vessels exhibited a 5% reduction in speed due to biofouling. The associated fuel penalties cost US Navy around $100 million annually. The estimated cost for removing organisms from ship-hulls, platforms and pipelines is in billions of dollars per year. With Zebra Mussels alone, the removal cost was about $5 billion in 2000. Biofouling causes surface corrosion and structural damage for aquatic infrastructures (e.g. oil platforms, water transportation and storage equipment, power plants), which leads to health and safety concerns. Reducing biofouling would also eradicate the potential transfer of marine community(s) from one location to another, thereby maintaining a natural ecosystem.
Naturally, the easiest way is the most brutal way, i.e., antifouling by simply killing off all the potentially fouling organisms near the surfaces. To this end, heavy metal based anti-fouling paints, such as tributyltin (TBT) and dibutyltin chloride, have been widely used. Equally predictably, the toxicity of these materials is not selective and their use has prompted serious environmental concerns. The use of TBT based coatings has been completely banned internationally by January 1, 2008. The environmental problems caused by other metal-based paints, containing copper, zinc, cadmium, chromium, have also limited their use in many countries. Organic biocides, such as SeaNine 211 and Irgarol 1051, have also been sough as alternatives; however, they cause the similar concerns of having non-specific biocidal effects beyond the targeted organisms.
The more difficult but environment-friendly way is to use non-toxic or less toxic natural antifoulants. But, because they don’t just kill off all organisms nearby, these natural antifoulants cannot be successfully applied without better understanding of how the biofouling takes place and how the antifoulants affect the interactions between the fouling organisms and the surfaces. Biofouling involves extremely complex phenomena and processes. There have been quite a bit of studies done for more medically oriented purposes, where the systems are nutritionally rich and probably more dominated by the cell-mediated affinity binding mechanisms. The situation may be quite different in biofouling occurring in natural water systems (rivers, lakes, and oceans).
We are interested in evaluating various natural antifoulants and our current effort is focused on their effects on the very beginning stage, i.e., first few hours, of microbial attachment. We will extend the study to the later stages as the study progresses. We expect this to be a rather long-term research before enough pieces of puzzles can be collected and put together for a clearer and workable picture for the environmental biofouling. We collaborate with Dr. Bi-minZhang Newby‘s research group on this project and are grateful for the current support by the Ohio Sea Grant program.
Experimental Diagram
For this study, flow chambers were used to observe the number of cells that attached to the surface of the glass chambers. The flasks that contained the solution and cells were connected to the chambers and different parameters were examined such as different species, flow rates, pH’s, etc. for better understanding of initial microbial attachment and initial stage of biofilm formation.
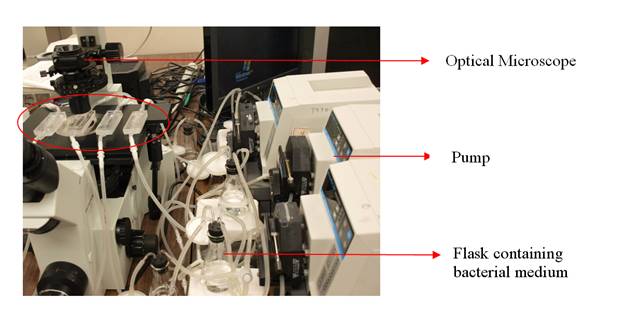 |
| Flow chamber system |
Formation of large aggregate structures formed in the systems containing Escherichia coli cells (the much smaller white specks) and 100 mg/L rhamnolipids. Rhamnolipids are glycolipid biosurfactants produced by bacteria, particularly Pseudomonas aeruginosa. Effects of rhamnolipids on initial attachment of P. aeruginosa, P. putida and E. coli on glass surface were investigated. It was observed that rhamnolipids prevented the attachment of P. aeruginosa significantly. The biosurfactants also lowered the attachment of P. putida but had so far unclear effects on E. coli. Also, large aggregates were sometimes observed to form in the P. putida and E. coli systems but not in the P. aeruginosa systems. The structures started out as smaller individual spheres and coalesced and grew into larger structures of cylindrical aggregates in less than 2 hours. We assumed these were micelles containing lipidic substances extracted from the cell suspensions.
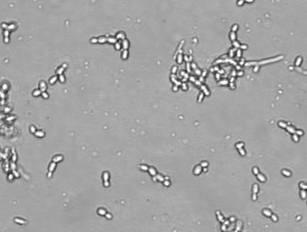 |
| Example of cell aggregates |