[Past Projects]
Dr. Randall Mitchell and Cynthia Yoder


Click here for more information about the Mitchell lab.



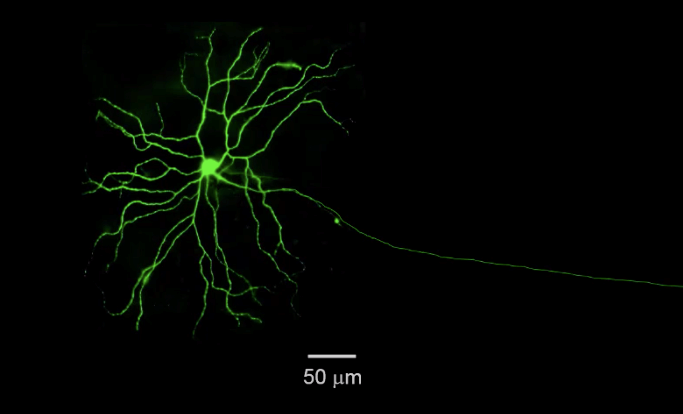
Our lab utilizes a procedure called Electroretinogram (ERG) to study the behavioral and physiological properties of the neural retina. This protocol has medical relevance and is routinely done by optometrists. It demonstrates the whole function of the retina and the function of several important cell populations. The A wave of the ERG is related to total output of light detecting cells (rods and cones). The B-wave of the ERG is related to the total output of the interneurons (or bipolar cells). These cells form a complex synapse that is needed for the transmission of visual information.
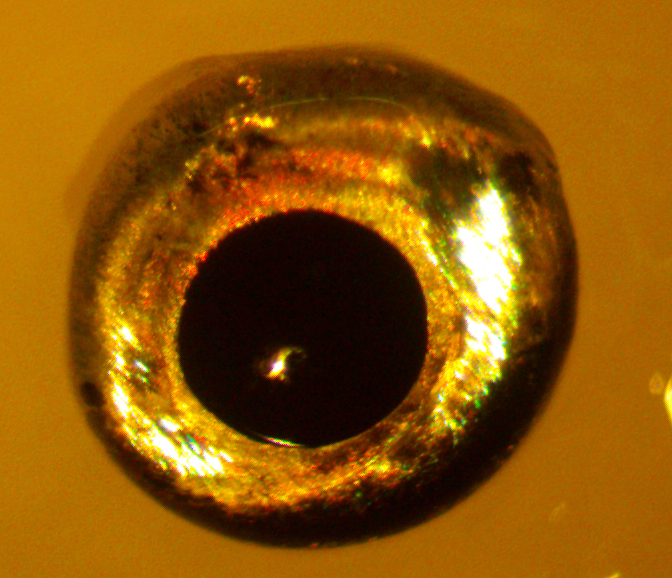
Our new project aims to classify how these connections are established and occur in zebrafish. Very few experiments have been done to measure the responsiveness of the zebrafish retina, so this approach is somewhat novel. The ease at which zebrafish can be genetically altered also opens more possible avenues for studying disease models and makes zebrafish a good model organism.
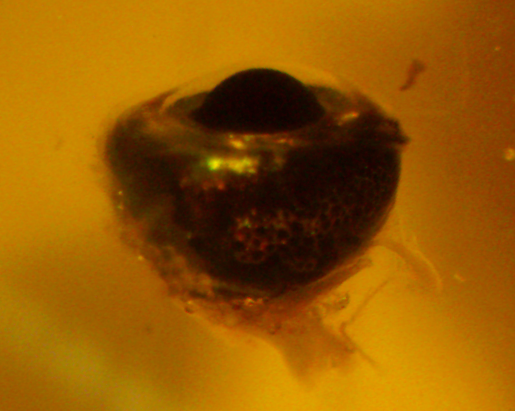
We are looking for students to help run ERG experiments and conduct the analysis of those experiments.