Heavy Element Chemistry
Theoretical modeling of bonding in lanthanide and actinide systems is very challenging. In strongly correlated systems with a partially filled f-shell, Coulombic repulsion tends to localize individual f-electrons at the atomic sites while hybridization with the ligand orbitals tends to delocalize the f-electrons. Actinide-ligand and lanthanide-ligand interactions are the most complex and polytypic in nature due to the increased nodality arising from the availability of the multiple lobes in the f-orbitals. Canonical molecular orbitals are typically very delocalized and difficult to interpret in terms of traditional Lewis bonding. In our research, we employ orbital composition analyses, electron localization algorithms, molecular orbital energy level diagrams, and energy decomposition schemes to disentangle the complex electronic structure of f-block compounds.
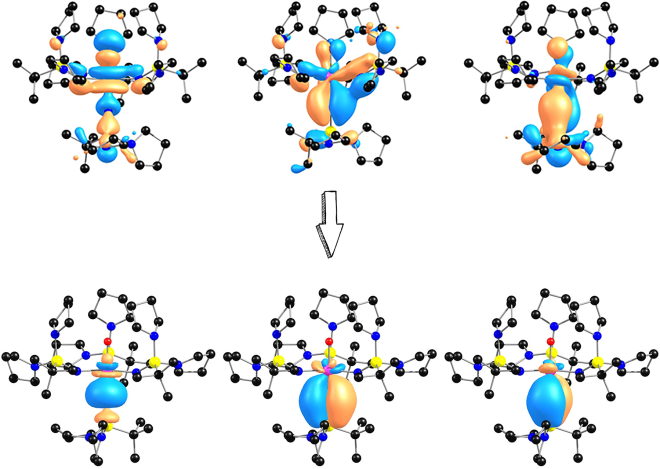
In addition to deciphering exotic multicenter Ln-L and An-L interactions, in our research we aim to answer fundamental questions related to the redox properties, thermodynamic stability, and reactivity of lanthanides and actinides in unusual oxidation states. Specifically, we are interested in understanding how the electronic structure of transuranic elements changes going from one extreme to another, from low to high oxidation states. This is particularly important for novel separation technologies and design of quantum information technologies.
On one hand, we are thrilled to elucidate highly reduced species, like AmII compounds, which should be stable because of their 5f7 electronic structure, but still have not been experimentally observed. Understanding this anomaly from the theoretical perspective will lead to a better understanding of the chemistry of Am and other actinides in general. On the other hand, we aim to develop theoretical guidelines that could be used to design heavy element compounds in high oxidation states, like TbIV and AmVI, wherein the metal orbitals are expected to mix with the ligand orbitals significantly, thus imposing a challenge for the description of their electronic structures.