Energy Storage
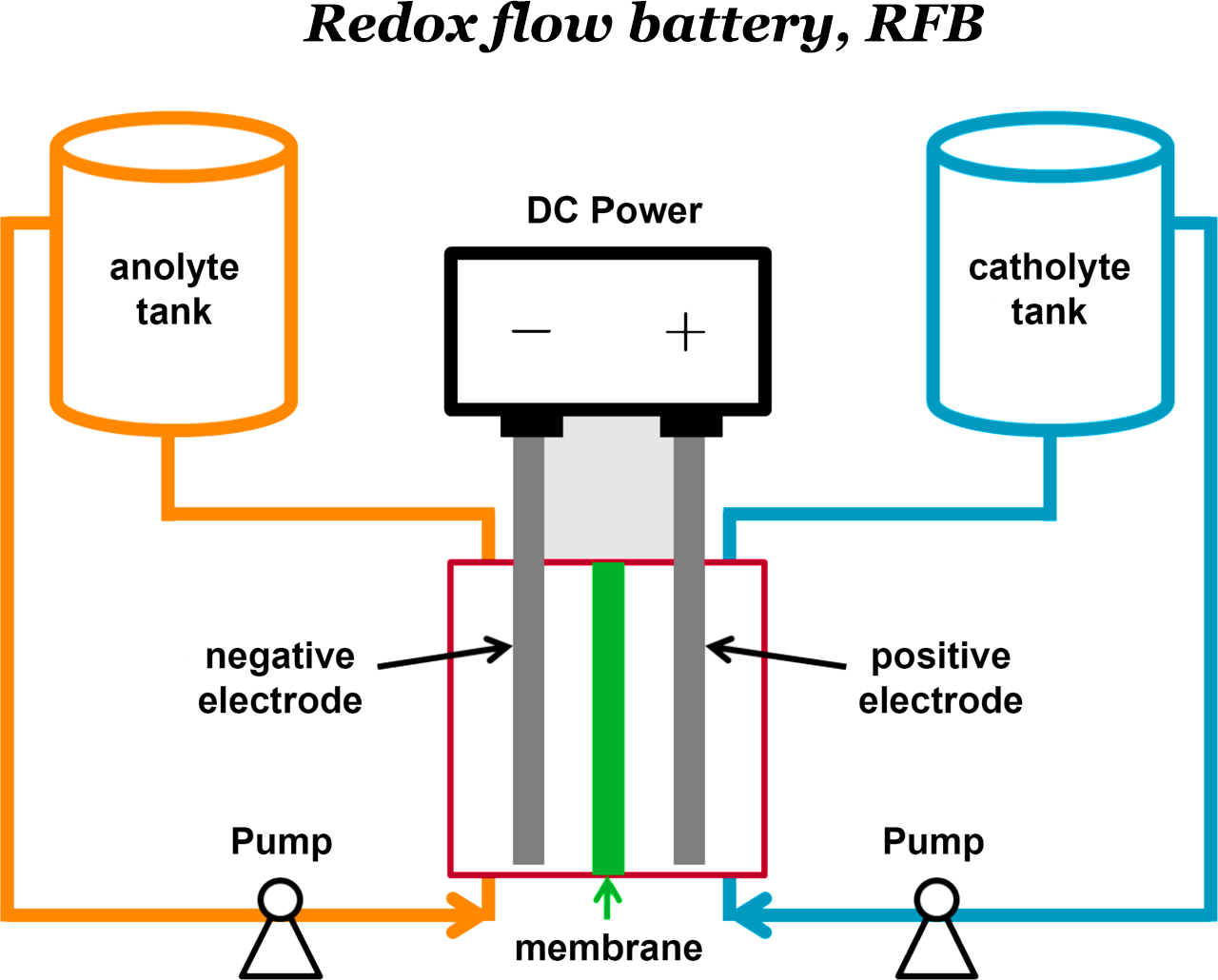
As human society moves toward more sustainable and renewable energy sources, new energy storage technologies need to be developed. Such systems must be scalable, cost effective, environmentally friendly, and possess a high energy density. One promising storage technology is the redox flow battery (RFB). Unlike traditional secondary batteries, where the capacity scales with electrode size, RFBs store energy in chemical species placed in tanks separate from the cell that facilitates power, and the voltage is generated between the respective electrodes immersed in those solutions.
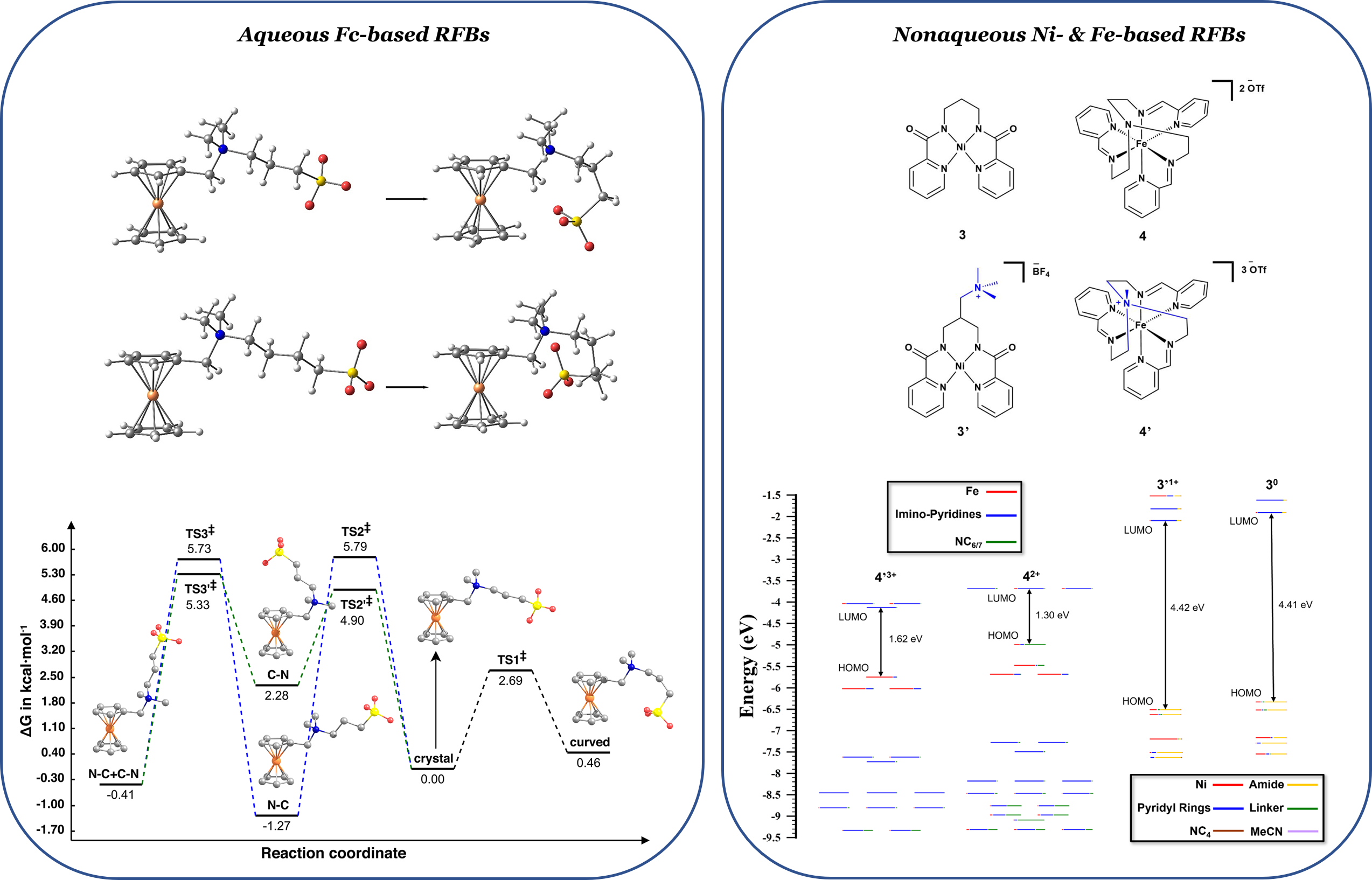
Redox potentials, solubility, stability and cyclability represent critical aspects of RFB operation. Further development of RFBs requires comprehensive theoretical understanding of these properties from the electronic structure perspective. It is highly desirable to improve the solubility and stability and understand the mechanisms of redox reactions of RFB compounds. A limited number of theoretically driven approaches in the current RFB research points to an urgent necessity to develop and employ theoretical models to explain properties of the RFB compounds as well as rationally guide the experiments. In our research, we address the chemical physics of RFBs from the microscopic level and complement theoretical instigations with experimental validation. We collaborate with inorganic chemists and electrochemists to help solve this energy consumption dilemma: the Ziegler and Boika groups at the University of Akron on ferrocene-based RFBs and the Davis group at Los Alamos National Laboratory on various Ni- and Fe-coordination charge carriers holding promise for RFBs.